Broken Federal Toxins Law Has 11% Chance of Being Fixed.
On December 16, the U.S. Food and Drug Administration (FDA) announced a proposed rule that allows soap and hygiene product manufacturers one year to prove “antibacterial” additives are safe and effective.
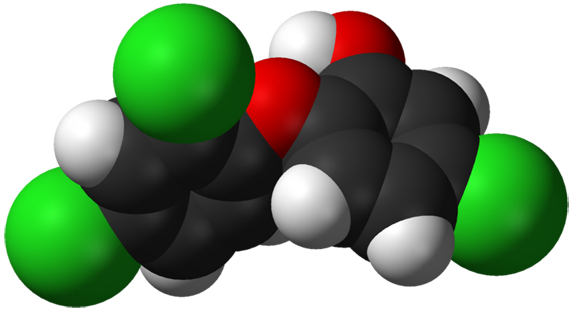
3D model of the “antibacterial” triclosan molecule. By Ben Mills [Public domain], via Wikimedia Commons
In this case, the two particular chemicals of concern are triclosan and triclocarban, which, according to research, disrupt hormones and body chemistry, increase antibiotic resistance, and harm aquatic ecosystems. They are used in soaps, toothpaste, detergents, mouthwash, almost anywhere you see the suspect claim of “antibacterial.” On product labels, one or the other is listed under “Drug Facts.” Sewage treatment plants do not remove them from human waste and, of course, consumers dispose of them as readily as they do kitchen scraps. The chemicals are now common in human urine and drinking water, and have been shown to have deleterious effects on aquatic organisms.
The FDA announcement about “antibacterial” chemicals comes soon after agency action on the excessive use of antibiotics in livestock, which itself followed more than three decades of warnings by health experts. (EarthDesk, December 13 & September 17). In that ruling, FDA’s proposed remedy was voluntary guidelines for the livestock industry; the industry was pleased. Even though the FDA website makes a strong case that “antibacterial” soap and hygiene products are a sham, it is far from certain the agency has a strong enough stomach to regulate product manufacturers beyond its current demand for proof.
On the environmental side, according to the FDA, it “and the Environmental Protection Agency (EPA) have been closely collaborating on science and regulatory issues related to triclosan.” EPA has chief environmental authority over the chemicals and regulates them as pesticides. In 2008, it announced a policy review will be forthcoming “in 2013, ten years earlier than originally planned,” even though health experts had already declared triclosan and triclocarban worthless to consumers for more than a decade.
A July 24, 2000 article by Kelly Woo in the Philadelphia Inquirer summarized well-established expert opinion: the chemicals likely increase antibiotic resistance, are unproven, and are not as good as old-fashioned soap and water:
“I think it’s totally a marketing ploy to feed into people’s fears about infection,” said Neil Fishman, director of the antimicrobial management program at the University of Pennsylvania Medical Center.
There may be specific situations where the products can be helpful – such as places where water is not readily available. But for everyday use at home, “no one has shown that they do any good,” said Stuart Levy, director of the Center for Adaptation Genetics and Drug Resistance at Tufts University. “Without a health benefit, the question is: Do we need them?
. . . Last month, the American Medical Association urged the government to increase regulation of antibacterial products, concluding that there was no scientific data for “any proven infection-fighting benefit.”
A March 25, 2005 article in The Montreal Gazette reported on the work of Dr. Rolf Halden, now of Arizona State University:
We’ve been using triclocarban for almost half a century at rates approaching one million pounds . . . We started looking and found [triclocarban] in the first sample we took, in the Baltimore region. Then we looked across the U.S. and found one or both antibacterial chemicals (triclocarban and triclosan) in 60 per cent of water supplies. . . [A]constant level of antibacterial chemicals may make disease-causing bacteria stronger, and more resistant to drugs.
Eight years later, he welcomes the FDA ruling. Halden told EarthDesk:
The FDA’s move is a prudent and important step toward (a) preserving the efficacy of clinically important antibiotics, (b) preventing unnecessary exposure of the general population to endocrine disrupting and potentially harmful chemicals, and (c) throttling back the increasing release and accumulation of antimicrobials in the environment.
Dr. John Kelly, microbial ecologist at Loyola University in Chicago, and Emma J. Rosi-Marshall, aquatic ecologist at Cary Institute of Ecosystem Studies, Millbrook, NY, studied the ecological effects of triclosan (TCS) exposure. The results of their work were published in the July 18 Environmental Science and Technology:
There was significant correlation between sediment TCS concentration and the proportion of cultivable benthic bacteria that were resistant to TCS, demonstrating that the levels of TCS present in these streams was affecting the native communities. An artificial stream experiment confirmed that TCS exposure could trigger increases in TCS resistance within cultivable benthic bacteria.
Kelly told EarthDesk:
I am supportive of the FDA’s decision to require manufacturers of products containing triclosan and triclocarban to demonstrate with data that these compounds are effective and safe. I would suggest that in assessing the safety of these compounds the potential environmental effects of these compounds be considered . . . I hope that our work represents a useful first step, but I believe that additional work on this topic is necessary to fully evaluate the safety of these compounds, not only in terms of human health but also in terms of ecosystem health.
Unfortunately, the larger question of safety is too often answered too late, if at all. The 1976 Federal Toxic Substances Control Act (TOSCA) does not protect the public and the environment from dangerous chemicals, as a New York Times April 18 editorial explained:
For the most part, the law requires the government to prove that a chemical is unsafe before it can be removed or kept off the market instead of requiring manufacturers to prove that their chemicals are safe before they can be sold and used. And it makes it hard for the Environmental Protection Agency to pry the information it needs to assess risk from the manufacturers or to require them to conduct tests.
Companies have to alert the E.P.A. before introducing new chemicals, but they don’t have to provide any safety data. It is up to the agency to find relevant scientific information elsewhere or use inexact computer modeling to estimate risk. The agency can only ask the company for data or require testing if it first proves there is a potential risk, which is hard to do without the company’s data.
The failure of the law can be read in these dismal statistics: since 1976, from a universe of chemicals that now numbers roughly 85,000, the agency has issued regulations to control only five existing chemicals.
Last April 10, the late Senator Frank Lautenberg of New Jersey and New York’s Kirsten Gilibrand introduced the Safe Chemicals Act of 2013 (S 696) to reform TOSCA. The bill had been a personal campaign of Senator Lautenberg’s through previous legislative incarnations dating to 2005. Said Gilibrand:
I was shocked to learn that in most instances, the federal government is unable to require safety testing of the chemicals used in the products my kids use every day. It’s outrageous that everything from car seats to my son’s dishware could be leaching hormone disrupting or cancer causing chemicals, but the EPA is virtually powerless to regulate them. We need to do better.
In his excellent summary of the bill and its political profile, Daniel Rosenberg wrote on his NRDC Switchboard blog that S 696 has the support of:
The American Academy of Pediatrics, the American Nurses Association, the National Medical Association, the Learning Disabilities Association, the Bladder Cancer Action Network, the Breast Cancer Fund, The American Fertility Association and, according to recent polling more than ¾ of the American public.
Opposing it:
Dow, DuPont, Exxon , Procter & Gamble, and other large chemical companies, along with dozens of other trade associations including the Grocery Manufacturers Association, the Toy Manufacturers, the Vinyl Institute, and the Chamber of Commerce.
The Times endorsement also noted the bill’s uphill battle:
The Safe Chemicals Act of 2013 would modernize and reform the law, mostly by requiring manufacturers to prove that a chemical is safe before it can be sold. It has more than two dozen Democratic co-sponsors but is opposed by the chemical industry and many Republicans, who argue that the E.P.A. already has enough power to regulate chemicals and simply needs to exercise it more effectively.
Dr. James Perrin, president of the American Academy of Pediatrics said of the Safe Chemicals Act:
As they grow and develop, children are especially vulnerable to the harmful effects of chemicals in the environment. Our current federal chemical management policy falls short for children. The Safe Chemicals Act of 2013 is a welcome and needed step toward protecting children and their families from harmful chemicals.
Because of intense industry and Republican opposition, Govtrack.us, which monitors and tracks federal bills, has given the Safe Chemicals Act an 11% chance of being enacted.












As a user we need to learn more about triclosan in cosmetics and in cleaning products to educate our self and spread the word! Thanks for the article…